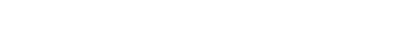Abbreviated Prescribing information
Product Name: Vyvanse 30 mg, 50 mg, and 70 mg hard capsules. Therapeutic indications: Vyvanse is indicated as part of a comprehensive treatment programme for attention deficit/hyperactivity disorder (ADHD) in
children aged 6 years and over when response to previous methylphenidate treatment is considered clinically inadequate. Vyvnse is not indicated in all children with ADHD and the decision to use the drug must be based
on a very thorough assessment of the severity and chronicity of the child’s symptoms in relation to the child’s age and potential for abuse, misuse or dininversion. Vyvanse is indicated as part of a comprehensive
treatment programme for attention deficit/ hyperactivity disorder (ADHD) in adults. Vyvanse is not indicated in all adult patients and the decision to use the medicinal product must take into consideration the profile of the
patient, including a thorough assessment of the severity and chronicity of the patient’s symptoms, the potential for abuse, misuse or diversion and clinical response to any previous pharmacotherapies for the treatment of
ADHD. Posology: Treatment must be initiated under the supervision of an appropriate specialist in behavioural disorders. Dosage should be individualised according to the therapeutic needs and response of the patient.
Careful dose titration is necessary at the start of treatment with Vyvanse. The starting dose is 30 mg taken once daily in the morning. When in the judgment of the clinician a lower dose is appropriate, patients may begin
treatment with 20 mg once daily in the morning. Vyvanse should be administered orally at the lowest effective dosage. The dose may be increased by 10 or 20 mg increments, at approximately weekly intervals. The
maximum recommended dose is 70 mg/day; higher doses have not been studied. Vyvanse may be taken with or without food. Special populations: Dexamfetamine clearance is reduced in the elderly, therefore dose
adjustment may be required. In patients with severe renal insufficiency, the maximum dose should not exceed 50 mg/day. Further dosage reduction should be considered in patients undergoing dialysis.
Lisdexamfetamine and dexamfetamine are not dialysable. Lisdexamfetamine dimesylate should not be used in children under the age of 6 years. Safety and efficacy in this age group has not been established.
Contraindications: Hypersensitivitytosympathomimetic amines or to any of the excipients, concomitant use of monoamine oxidase inhibitors (MAOI) or within 14 days after MAOI treatment, hyperthyroidism or
thyrotoxicosis, agitated states, symptomatic cardiovascular disease, advanced arteriosclerosis, moderate to severe hypertension, and glaucoma. Warnings and Precautions: Abuse and dependence: Stimulants including
Vyvanse have a potential for abuse, misuse, dependence, or diversion for nontherapeutic uses that physicians should consider when prescribing this product. Stimulants should be prescribed cautiously to patients with a
history of substance abuse or dependence. Cardiovascular adverse events: Sudden death has been reported in children and adolescents taking CNS stimulants, including those with structural cardiac abnormalities or
other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural
cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug. Adults
have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such
abnormalities should also generally not be treated with stimulant drugs. All patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying
medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia. Vyvanse should be
used with caution in patients with prolongation of the QTc interval, in patients treated with drugs affecting the QTc interval, or in patients with relevant pre-existing cardiac disease or electrolyte disturbances. Patients who
develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation. Psychiatric adverse
events: Administration of stimulants may exacerbate symptoms of behaviour disturbance and thought disorder in patients with pre-existing psychotic disorders. Particular care should be taken in using stimulants to treat
ADHD patients with comorbid bipolar disorder because of concern for possible induction of mixed/mani episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms
should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual
doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. Patients beginning treatment for ADHD should be monitored
for the appearance of or worsening of aggressive behaviour or hostility. Clinical evaluation for tics and Tourette’s syndrome should precede use of stimulant medications. Long-term suppression of growth (height and
weight): In Children aged 6 years and over, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining weight as expected may need to have their treatment interrupted.
Height, weight, and appetite should be recorded at least 6-monthly. In adults, Stimulants have been associated with weight loss. Weight should be monitored during treatment with stimulants, and patients who are losing
weight may need to have their treatment interrupted. Seizures: There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizure, in patients with prior EEG
abnormalities in absence of seizures, and very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of new onset or worsening seizures, the drug should be discontinued.
Interaction with other medicinal products and other forms of interaction: In vitro experiments with human microsomes indicate minor inhibition of CYP2D6 by amfetamine and minor inhibition of CYP1A2, 2D6, and 3A4 by
one or more metabolites. Although the clinical significance of this interaction is likely to be minimal, consideration should be given when medications metabolised by these pathways are administered. Ascorbic acid and
other agents and conditions (thiazide diuretics, diets high in animal protein, diabetes, respiratory acidosis) that acidify urine increase urinary excretion and decrease the half-life of amfetamine. Sodium bicarbonate and
other agents and conditions (diets high in fruits and vegetables, urinary tract infections and vomiting) that alkalinise urine decrease urinary excretion and extend the half-life of amfetamine. Serotonin syndrome has rarely
occurred in association with the use of amphetamines such as Vyvanse, when given in conjunction with serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline
reuptake inhibitors (SNRIs). It has also been reported in association with overdose of amphetamines, including Vyvanse. Amfetamines may decrease the effectiveness of guanethidine or other antihypertensive
medications. Amfetamines potentiate the analgesic effect of narcotic analgesics. Chlorpromazine blocks dopamine and norepinephrine receptors, thus inhibiting the central stimulant effects of amfetamines. Haloperidol
blocks dopamine receptors, thus inhibiting the central stimulant effects of amfetamines. The anorectic and stimulatory effects of amfetamines may be inhibited by lithium carbonate. Amfetamines can cause a significant
elevation in plasma corticosteroid levels. This increase is greatest in the evening. Amfetamine may interfere with urinary steroid determinations. Pregnancy and lactation: The physician should discuss Vyvanse treatment
with female patients who have started menstruation. The physician should discuss Vyvanse treatment in the context of potential pregnancy or lactation with female patients of child-bearing potential. Vyvanse should only
be used during pregnancy if the potential benefit justifies the potential risk to the foetus. Amfetamines are excreted in human milk. Vyvanse should not be used during breast-feeding. The effect of Vyvanse on human
fertility has not been investigated. Effects on ability to drive and use machines: Vyvanse can cause dizziness, drowsiness and visual disturbances including difficulties with accommodation and blurred vision. These could
have a moderate influence on the ability to drive and use machines. Patients should be warned of these possible effects and advised that if affected, they should avoid potentially hazardous activities such as driving or
operating machinery. Undesirable effects: Very common adverse reactions include decreased appetite, insomnia, dry mouth, headache, upper abdominal pain, and weight decreased. Marketing Authorization Holder:
Takeda Pharmaceuticals International AG Ireland Branch, Dublin 2, Ireland. For additional Important Safety Information, please see the full summary of product characteristics for Vyvanse, Vyvanse EU SmPC 2022.










 All are adults
All are adults
 Mostly adults with some children
Mostly adults with some children
 Evenly split between adults and children
Evenly split between adults and children
 Mostly children with some adults
Mostly children with some adults
 All are children
All are children




 Stimulant medications
Stimulant medications
 Non-stimulant medications
Non-stimulant medications




 True
True
 False
False




 True
True
 False
False




 True
True
 False
False




 True
True
 False
False




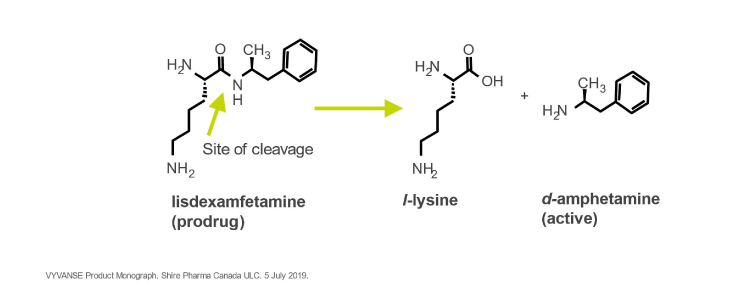
 Superior
Superior
 Inferior
Inferior
 Equivalent
Equivalent